
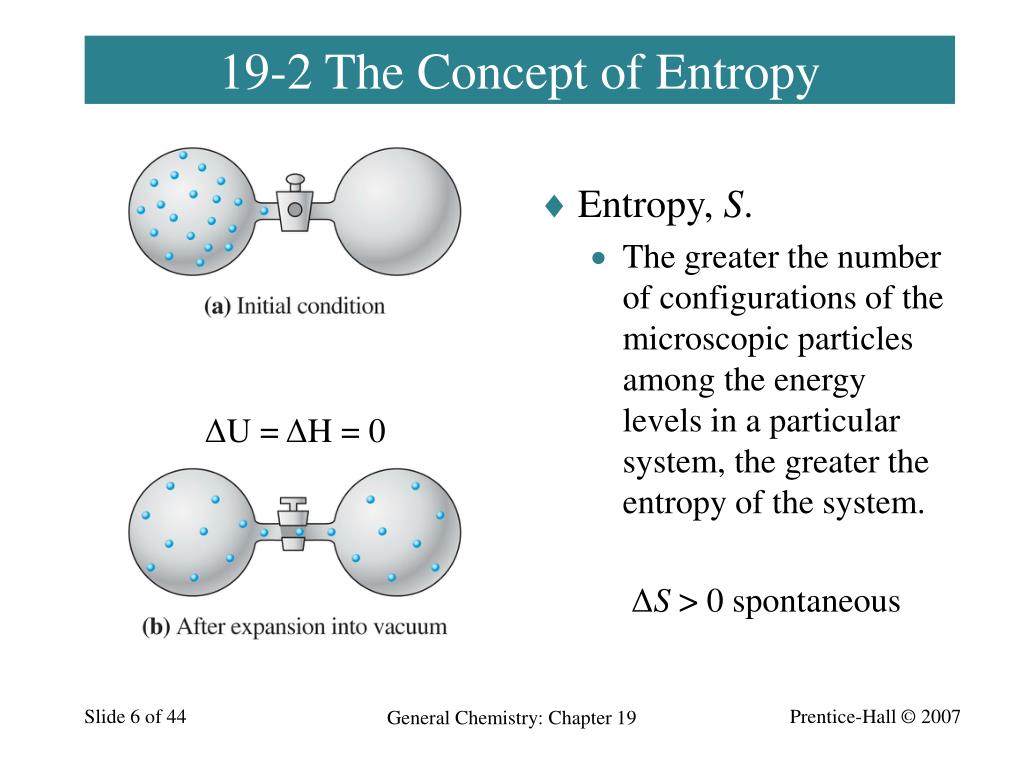
The transfer of heat goes from the hot object to the cold object. For example, if we bring a hot object into contact with a cold object, the hot object cools down and the cold object heats up until an equilibrium is reached. However, we can imagine thermodynamic processes which would conserve energy but which never occur in nature. The first law of thermodynamics defines the relationship between the various forms of kinetic and potential energy present in a system, the work which the system can perform and the transfer of heat. The law states that energy is conserved in all thermodynamic processes. In aerodynamics, the thermodynamics of a gas obviously plays an important role in the analysis of propulsion systems. Thermodynamics deals only with the large scale response of a system which we can observe and measure in experiments. Thermodynamics is a branch of physics which deals with the energy and work of a system. The sequence of cycles is repeated using heat reservoirs at appropriate temperatures, until the two bodies arrive at a common temperature T f.Home > Beginners Guide to Aeronautics Second Law – Entropy Second Law of Thermodynamics Furthermore, the temperature of the hot body is reduced to T 1 − δ T 1 and that of cold body rises to T 2 + δ T 2, thus decreasing the difference between their temperatures. As a consequence, the sum of the entropy changes in the bodies is also zero. In this whole process, the engine and the auxilliary reservoirs undergo a reversible process, so their total change in entropy is zero. This heat is then reversibly transferred to the cold body at T 2. Now, an infinitesimal amount of heat dQ 1 is reversibly transferred from the hot body to the reservoir at T 1 − δ T 1, a reversible heat cycle is run, which outputs work dW and rejects heat d Q 2 = d Q 1 − d W to the reservoir at T 2 + δ T 2. Now, assume that we have another pair of reservoirs, which differ from the initial pair by infinitesimally different temperatures, denoted as T 1 − δ T 1 and T 2 + δ T 2, where δ T i > 0. To visualize one such infinitesimal heat cycle-say, the first one-we have prepared the two bodies in initial states using reservoirs at temperatures T 1 and T 2. The envisaged reversible step requires the presence of a heat engine, a reversible work source, and a set of auxiliary heat reservoirs. We argue below that T F > T f holds not only for the case with algebraic means but also in general. Therefore, we can say that the condition T F > T f directly implies Δ S > 0. (3) may be reexpressed as Δ S = ( C 1 + C 2 ) ln ( T F / T f ). 14 Now, T f is determined by the reversibility condition: C 1 ln ( T f / T 1 ) + C 2 ln ( T f / T 2 ) = 0, yielding T f = T 1 α T 2 1 − α. 1,12,13 This may be achieved by introducing a heat engine and running infinitesimal, reversible heat cycles that gradually reduce the temperature difference between the two bodies, until the two bodies obtain a common temperature T f. More precisely, consider a reversible process that extracts work from the two bodies initially at temperatures T 1 and T 2. 2–11 However, the fact that one of the means in the above comparison, T 1 α T 2 1 − α, is also the final common temperature of the two bodies when subjected to the process of reversible work extraction, seems to have escaped attention in the literature so far. There has been previous discussion around this apparent correspondence between physical laws and mathematical facts such as these inequalities. In this case, the proof of the inequality Δ S > 0 rests on the inequality between weighted arithmetic and geometric means, given by α T 1 + ( 1 − α ) T 2 > T 1 α T 2 1 − α, for T 1 ≠ T 2.


 0 kommentar(er)
0 kommentar(er)
